NEWS
You can follow the latest news of MicroViable in this section
Microviable will be attending Drug Discovery Innovation Programme 2024
Microviable is excited to join 24th Drug Discovery Innovation Programme that will take place on 19-20 September in Barcelona. Claudio Hidalgo, CEO at Microviable, will participate as a speaker at the event. Drug Discovery Innovation [...]
Microviable receives best company award at Keiretsu Forum event
Last week we presented Microviable Therapeutics at the online investors chapter of Keiretsu Forum and we got awarded as the best company, by the pool of investors present. Keiretsu Forum is a global investment community [...]
Microviable participates in a research that has demonstrated that certain microbiota bacteria can be used as new probiotics to improve physical performance.
The work, led by a joint IPLA-University of Oviedo research group and the company Microviable Therapeutics, with Susana Delgado, from IPLA-CSIC, as the responsible researcher, has managed to identify and select bacteria in the microbiota [...]
Microviable will be presenting at Bio€quity Europe. San Sebastián
Claudio Hidalgo Cantabrana, CEO at Microviable Therapeutics, and our CBO, Rafael Permuy, will be attending Bio€quity Europe event in San Sebastián (Spain) from May 12th to 14th and May 21, 22 (virtual). On Tuesday 14th [...]
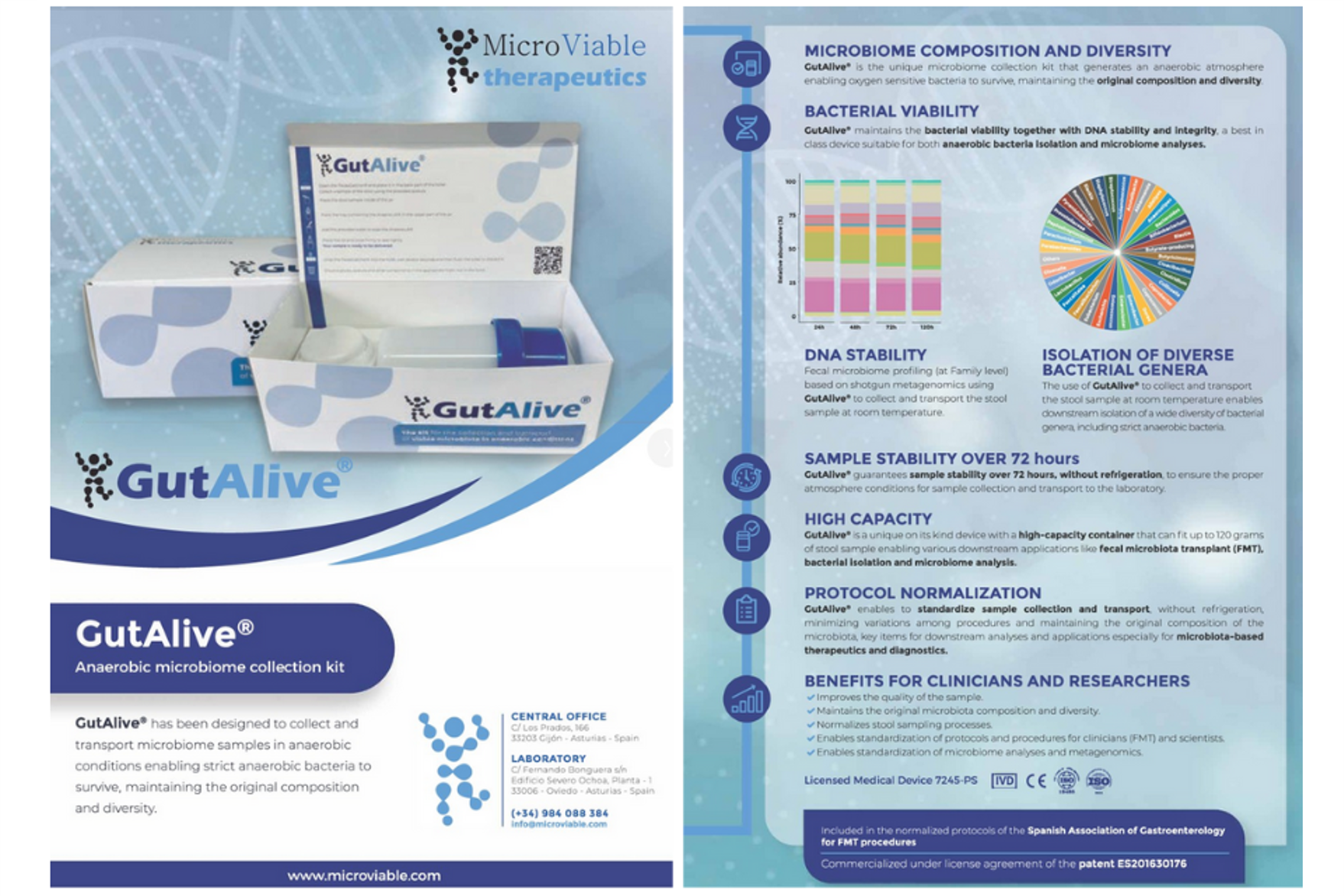
Microviable signed an agreement with Deltalab for the distribution of GutAlive kit, designed for the collection and transport of microbiota.
Microviable Therapeutics has signed a collaboration agreement with the Healthcare & Lifescience division of Deltalab (member of SCGP) for the international distribution of GutAlive® kit for the collection and transport of microbiota. Thanks to [...]
Microviable will be attending Bio€quity Europe. San Sebastián
Claudio Hidalgo Cantabrana, CEO at Microviable Therapeutics, and our CBO, Rafael Permuy, will be attending Bio€quity Europe event in San Sebastián (Spain) from May 12th to 14th and May 21, 22 (virtual). Microviable Team joins [...]