NEWS
You can follow the latest news of MicroViable in this section
Microviable will be attending 8th Microbiome Movement- Drug Development Summit Europe
Claudio Hidalgo Cantabrana, PhD, CEO at Microviable, and Pilar Manrique, PhD, Research Scientist, will be attending the 8th Microbiome Movement - Drug Development Summit Europe which will take place from 30 January to 1 February 2024 [...]
Wishing you all a Merry Christmas and a prosperous New Year!
From Microviable we want to express our most sincere wishes for you and your loved ones this holiday season. May this Christmas your days be filled with laughter, your home with love and your heart [...]
The Microbiota and Cancer: Exploring the Link between Microbial Composition and Oncogenesis
The microbiota plays a crucial role in human health, directly influencing digestive functions, protection against pathogens, modulation of the immune system, and the production of essential vitamins and metabolites. In relation to oncological processes, numerous [...]
Abelardo Margolles, co-founder and scientific advisor of Microviable among the Highly Cited Researchers 2023.
Clarivate a research organization and global expert in data insights and collection, recognizes the true pioneers in their fields over the last decade, demonstrated by the production of multiple highly-cited papers that rank in the [...]
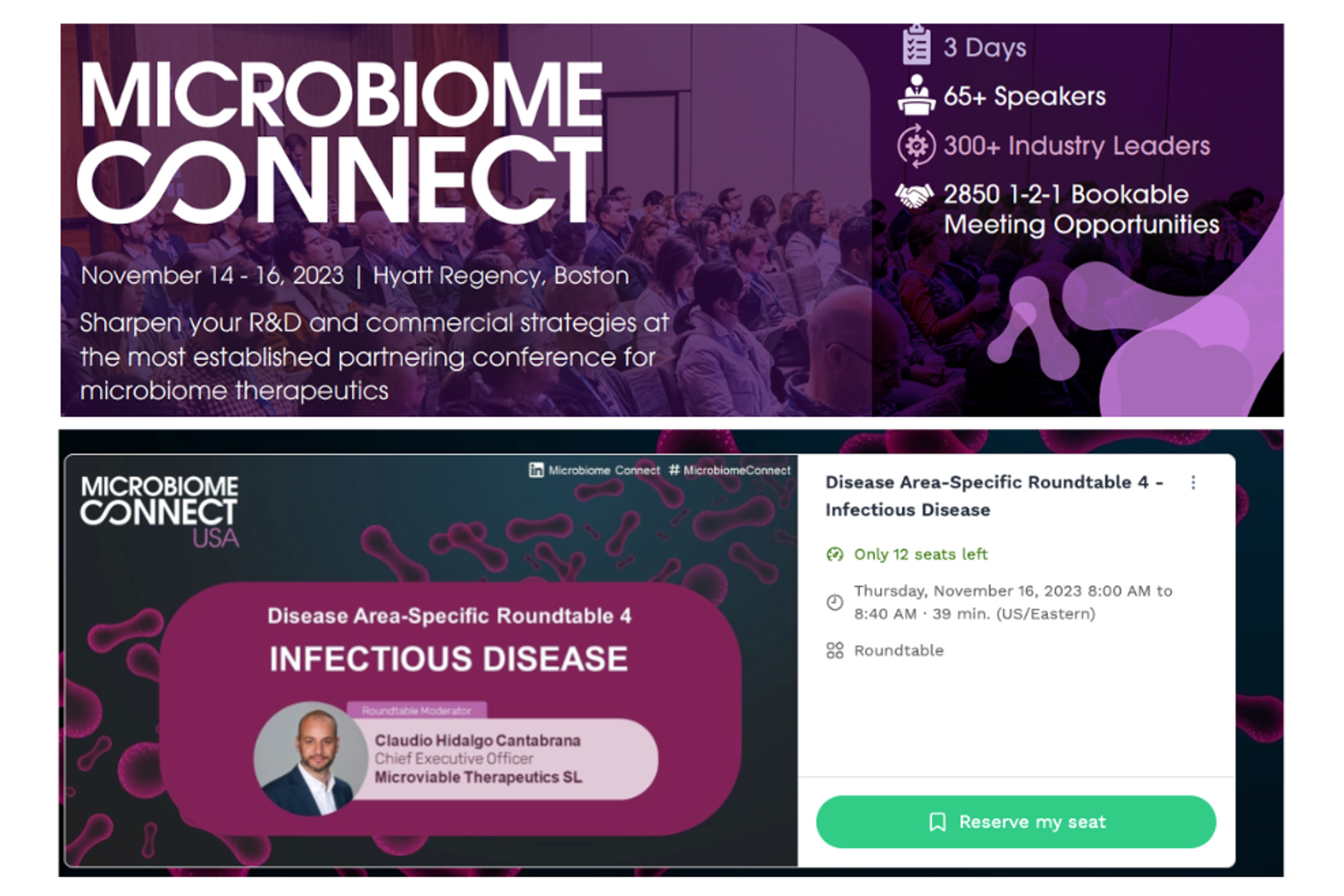
Microviable Therapeutics will be presenting at Microbiome Connect USA
Microviable Therapeutics will participate in Microbiome Connect USA that will take place from November 14th to 16th in Boston. Claudio Hidalgo Cantabrana, CEO at Microviable, will join a like-minded community of 300 scientists and entrepreneurs [...]
Standardizing the sampling and transport of the fecal specimens to the lab
The vast majority of microbiome studies failed to address core methodological aspects including the use of anaerobic conditions, sample volume, storage etc. with a lack of protocol normalization and standardization (1). Following the agreement [...]