Microviable Therapeutics has signed a collaboration agreement with the Healthcare & Lifescience division of Deltalab (member of SCGP) for the international distribution of GutAlive® kit for the collection and transport of microbiota.
Thanks to this agreement, Microviable will expand the international market of the product continuing to grow, surpassing its current presence in more than 25 countries.
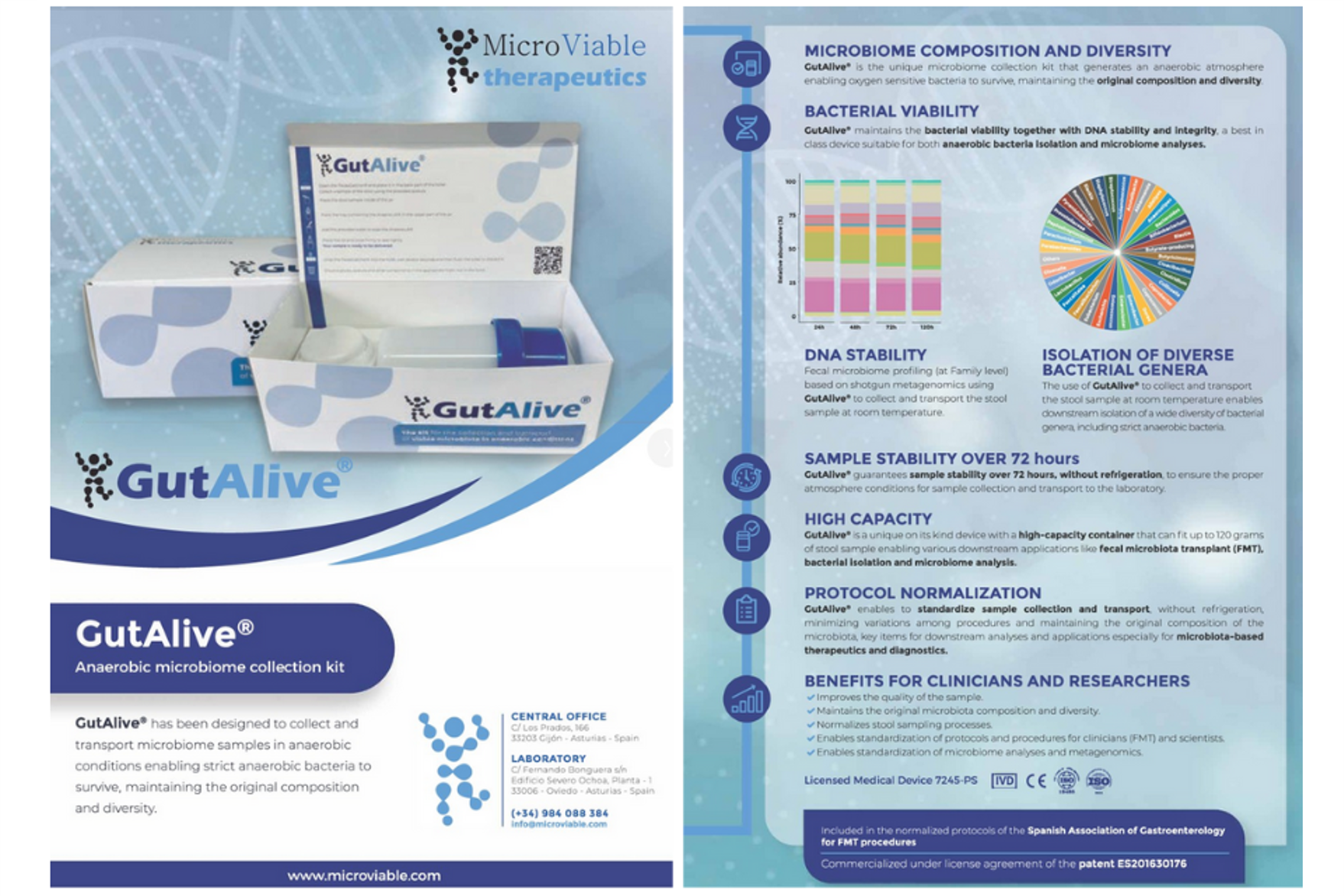
GutAlive® is the unique anaerobic stool collection device that ensures sample stability and microbiota viability, preserving the original composition and diversity by creating an anaerobic atmosphere inside. It also preserves DNA integrity thanks to bacterial cell viability.
GutAlive® is being used for bacterial isolation, including strict anaerobic bacteria, FMT procedures and for microbiome analysis through metagenomics sequencing. Microviable is currently use at several hospitals, research centers and biotechnological companies. It is also included in the normalized protocols of the Spanish Association oGastroenterology for FMT procedures.