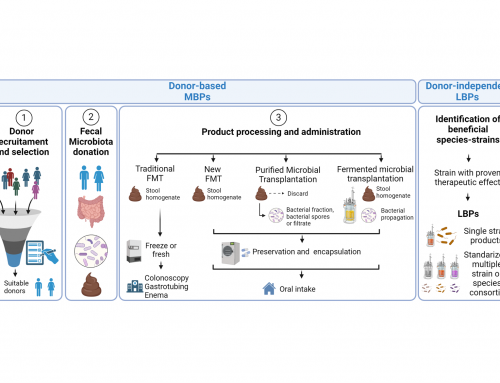
Microviable Therapeutics SL, December 22nd, 2021, a preclinical stage biotechnology company developing microbiota-based products today announced that it has completed preclinical analyses on safety and colonization capability of their unique Human Purified Microbiota (HPMTM) product.
The proprietary technology developed over the last years has enabled Microviable to obtain purified and concentrated microbiota from healthy donors without the need of propagating microbes, as well as the capability to store the HPMTM in viable conditions for long-term. Now, with the latest preclinical data in antibiotic-treated mice models (data not published), Microviable has shown the safeness of this product. Additionally, these results have also validated the technology that enables to store HPMTM for long-term in viable conditions without disrupting the original composition of the microbiota. Noteworthy, HPMTM can partially colonize and reshape the mice gut over the first days with a single dose administered.
Microviable continues to generate promising data on their proprietary technology developing complete microbiota-based products to bring it into the clinical stage in 2022. The company is seeking to start Phase1/2a clinical trials in the second quarter of 2022 to provide novel solutions into neurological and inflammatory disorders.
“Microbiota-based products are still not properly regulated but growing evidence by the scientific community, both in academia and industry, has built strong scientific record on the benefits of using gut microbes to improve human health. By using HPMTM we will be able to use complete healthy microbiota to reshape the gut dysbiosis and associate immune disorders existing in several diseases, without the need of propagating fastidious bacteria or limiting live bacterial products to a handful of species that can be grown at industrial scale”, said Claudio Hidalgo Cantabrana, Chief Executive Officer at Microviable Therapeutics SL.