Gijón, 21st July 2022, Microviable Therapeutics SL, a biotechnology company in preclinical stage developing novel microbiome-derived biotherapeutic products, announced a research agreement with the University Medical Center of Utrecht (The Netherlands) and Artizan Biosciences Inc. (New Haven, USA) to elucidate the gut microbiota implications in the success and side effects of immune checkpoint inhibition (ICI) therapies for cancer treatments.
The intestinal microbiota composition plays an important role in ICI response based on the crosstalk between the gut bacteria and the immune system with the subsequent impact on T-cell response. Immunostimulatory bacterial species play a key role in the effectiveness of ICI therapy and thereby cancer survival. Microviable´s proprietary platform for strict anaerobic bacteria isolation (SABITM) will enable to identify, isolate, and characterize the key microbes involved in successful ICI response. Coupled with the live biotherapeutic products (LBPs) development pipelinewill open new avenues for novel microbiota-based biotherapeutic products to improve cancer treatments.
“We are delightedabout this multi-year research collaboration and the tremendous potential to advance the science behind gut microbiota and ICI response, while expanding Microviable´s platform into immuno-oncology ”, said Claudio Hidalgo Cantabrana, PhD, co-founder and CEO of Microviable Therapeutics.
We look forward to start working with UMC Utrecht and Artizan Biosciences, a solid team to identify novel solutions that can improve cancer patient outcomes and reduce the side effects of ICI therapies.
About Microviable Therapeutics
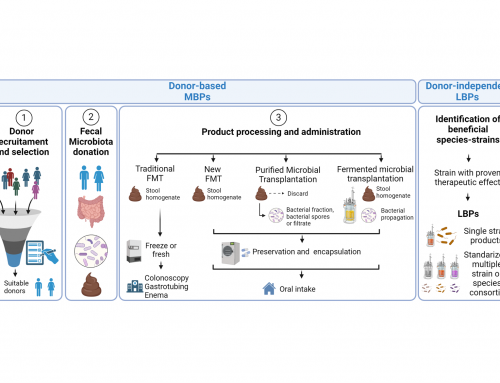
Microviable Therapeutics is developing novel technologies and biotherapeutic products derived from the gut microbiota to impact various diseases.The company´sproprietary technology enables to generatea unique in its kind product,a novel whole ecosystem biotherapeutic product named Human Purified Microbiota (HPM®). This exclusive microbiome product will be in the clinic by 2023. Moreover, the strict anaerobic bacterial isolation (SABITM) platform empowers the isolation and characterization of hundreds of unique bacterial isolates, allowingthe development of live biotherapeutic products (LBPs)based on rationally designed and defined bacterial consortia. These two modalities of microbiome-derived biotherapeutic products are applicable to a broad range of diseases including infectious diseases (pathogen exclusion), skin disorders (immune modulation), cancer and immune-oncology, and neurodegenerative diseases.
Contact:
Claudio Hidalgo Cantabrana, PhD
984088384
info@microviable.com