ADVANCED MICROBIOTA DRUG DEVELOPMENT PLATFORM
We are harnessing the complete microbiota ecosystem to develop novel biological drugs and microbiome therapies for various diseases.
Complete Microbiota Ecosystem Products
Microviable Therapeutics’ lead product represent the complete gut microbiota ecosystem with high diversity and full functionality to be harnessed as a biological drug. This unique complete microbiota ecosystem product is a non-propagated, donor-derived, high-diversity and high-richness product generated with our proprietary technology obtaining human purified microbiota (HPM®) in viable conditions.
Our lead candidate MVT-201, is an orally administered capsule generated under cGMP conditions representing the complete gut microbiota of a clinically validated healthy donor and will get into the clinical trials in 2024 for undisclosed targets.

Microbiota Therapies
Our capability to obtain donor-derived human purified microbiota enable us to perform microbiota therapies as precision medicine.
The gut microbiota of a specific individual can be purified and use for autologous or heterologous applications, either in native conditions or rationally modified for the depletion or enrichment of the desired bacterial populations.
These microbiome therapies are being co-developed in partnership with Inmunomet Intolerancias y Disbiosis
Microbiota Therapies
Our capability to obtain donor-derived human purified microbiota enable us to perform microbiota therapies as personalized medicine.
The gut microbiota of a specific individual can be purified and use for autologous or heterologous applications, either in native conditions or rationally modified for the depletion or enrichment of the desired bacterial populations.
These microbiome therapies are being co-developed in partnership with Inmunomet Intolerancias y Disbiosis

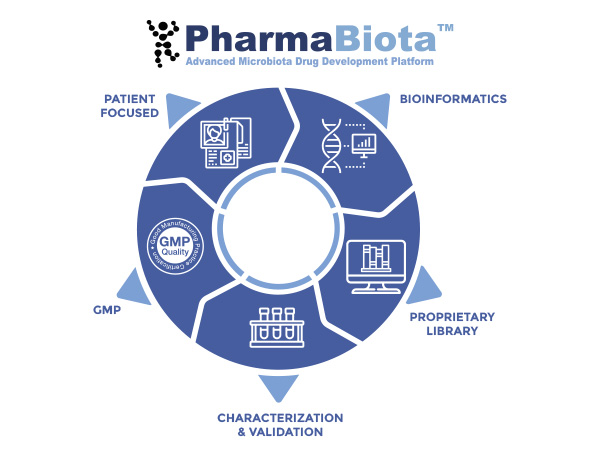
PharmaBiota® Platform
Our Advance Microbiota Drug Development Platform enables the generation of a novel category of biotherapeutic products based on commensal bacteria developing personalized drugs for unmet clinical needs.
Our large and diverse proprietary bacterial culture collection together with our bioinformatics platform enable the discovery of novel microbiota-derived biological drugs, tailored with our in house characterization pipeline and cGMP production.
| Patent No. | Title | Applicant | Issue date |
|---|---|---|---|
| ES201630176 | Device for microbiota collection and transport in anaerobic condition | CSIC | July 10, 2018 |
| EP19383077 | Tools and methods to detect and isolate colibactin producing bacteria | CSIC | Pending |
| Trade Secret (ref.: 2203) | Methods for long-term preservation of gut microbiota in viable conditions | CSIC-MVT | April 13, 2021 |
| Trade mark | PharmaBiota® | Microviable Therapeutics | December 02, 2022 |
| Trade mark | HiPM® – Human Purified Microbiota | Microviable Therapeutics | June 08, 2022 |
| Trade mark | GutAlive® | Microviable Therapeutics | January 23, 2018 |
| Trade mark | Microviable Therapeutics® | Microviable Therapeutics | April 26, 2017 |
Patent No.: ES201630176
Title: Device for microbiota collection and transport in anaerobic condition
Applicant: CSIC
Issue date: July 10, 2018
Patent No.: EP19383077
Title: Tools and methods to detect and isolate colibactin producing bacteria
Applicant: CSIC
Issue date: Pending
Patent No.: Trade Secret (ref.: 2203)
Title: Methods for long-term preservation of gut microbiota in viable conditions
Applicant:CSIC-MVT
Issue date: April 13, 2021
Patent No.: Trade mark
Title: PharmaBiota®
Applicant: Microviable Therapeutics
Issue date: December 02, 2022
Patent No.: Trade mark
Title: HiPM® – Human Purified Microbiota
Applicant: Microviable Therapeutics
Issue date: June 08, 2022
Patent No.: Trade mark
Title: GutAlive®
Applicant: Microviable Therapeutics
Issue date: January 23, 2018
Patent No.: Trade mark
Title: Microviable Therapeutics®
Applicant: Microviable Therapeutics
Issue date: April 26, 2017
* All the disclosed IP has been licensed with exclusive rights to Microviable Therapeutics SL.
